Abstract
Background: Persons with mild or moderate hemophilia A (HA) account for 40-52% of all persons with HA (PwHA) but are under-represented in scientific literature. To address this gap, we utilized a novel, patient-centric approach developed by PicnicHealth to create a longitudinal healthcare database from persons with mild or moderate HA in the United States (US). This study integrates data collected during routine clinical care from various providers and sites along with patient-reported outcomes (PROs) on an online record management platform to provide an in-depth characterization of patients' disease journey.
Methods: PicnicHealth HA study recruitment commenced in June 2020. Eligible PwHA had a documented HA diagnosis. Severity status was confirmed based on physician-reported severity in provider notes or reported baseline factor (F)VIII level (>5-50% mild, 1-5% moderate); the lowest FVIII value was used if notes and baseline levels were inconsistent. Data elements from structured text (e.g. medication lists) as well as disease-specific elements from narrative text were captured from patients' electronic health records and linked to self-reported data. PROs were collected biweekly via an online questionnaire for a subset of PwHA. Ethics approval was obtained. Further details on the PicnicHealth platform will be reported separately.
Quality control was assessed via inter-abstractor agreement on outputs with physician review. Descriptive analyses were performed to summarize cohort characteristics and demonstrate the breadth and completeness of the data. Cohort characteristics were compared with mild and moderate PwHA data from the Center for Disease Control and Prevention (CDC) Community Counts, a public health monitoring program for people with bleeding disorders.
Results: As of June 2021, 143 PwHA had signed up to the study, of whom 104 (65 [62.5%] with mild HA; 39 [37.5%] with moderate HA) met the eligibility criteria for enrollment. PwHA were recruited from 32 US states. Among those enrolled, 22.1% (23/104) were female. Median age at enrollment was 29 years (interquartile range [IQR]: 22-42). Age at diagnosis was available for 55 (52.9%) PwHA; the median was 3 years (IQR 0-12). Notably, males were diagnosed at a younger age (2 [0-10] years) than females (18 [5-35] years). Among PwHA aged ≥20 years (n=83), 59 had valid body mass index (BMI) measures, of whom 44 were obese (n=28, 47.4%) or overweight (n=16, 27.1%). These cohort characteristics were generally in line with those reported by the CDC's Community Counts, e.g. by age, BMI and ethnicity distributions, except for the higher proportion of female PwHA recruited in the present study (Table).
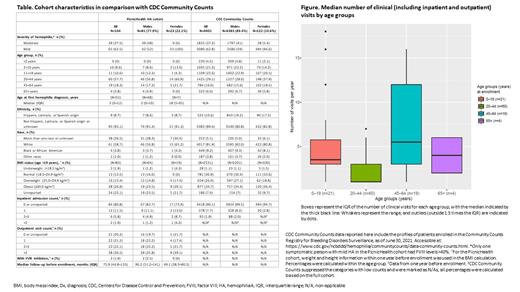
Medical records were available over a median (IQR) of 6.3 (3.7-11.1) years from 16 (8-28) healthcare providers of six (3-10) care sites for the cohort before the enrollment dates (Table). In total, data were collected from 588 care sites and 1963 providers, including 234 hematology and 1729 non-hematology providers, with specialties including radiology, family medicine, pediatrics, and orthopedics. In the year before enrollment, 83 PwHA had outpatient visits, with a median of three visits for the cohort; 20 PwHA had inpatient admissions (Table). Inpatient admission and outpatient visit frequencies in the year before enrollment were lowest in the 20-44 years age group (median [IQR] 1 [1-3]) (Figure).
PROs were collected prospectively via biweekly requests in 25 PwHA starting from Feb 25, 2021 with the latest data collected in June 2021. On average, the response rate was 90.3% (150/166 requests). Of the 25, 10 (40.0%) responded to all questions at the expected time, nine (36.0%) missed one response, and six (24.0%) had more than one response missing.
Conclusions: The patient-centric data collection methods implemented in this study provide a new approach to building cohorts for observational studies for PwHA. PicnicHealth combines routine clinical care data and PROs, and preliminary results suggest validity when compared with an existing data set. Participants have access to all records collected, organized in a medical timeline and shareable with care providers, potentially alleviating the burden of care coordination for the patient. The result is a patient-centric approach to data that benefits participants while providing needed data on groups traditionally under-represented in real-word evidence and traditional PwHA cohorts.
Witkop: National Hemophilia Foundation: Current Employment; Roche Advisory Panel: Consultancy. Xu: F. Hoffmann-La Roche AG: Current Employment. Hanson: PicnicHealth: Current Employment, Current holder of stock options in a privately-held company. Ofori-Asenso: F. Hoffmann-La Roche Ltd: Current Employment. Ko: Genentech, Inc.: Current Employment; Genentech, Inc.-Roche: Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Cibelli: PicnicHealth: Current Employment. Aizenas: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Skinner: Institute for Policy Advancement Ltd: Current Employment; National Hemophilia Foundation: Consultancy; IPA Ltd.: Current holder of individual stocks in a privately-held company; BioMarin: Honoraria, Research Funding; Freeline: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Takeda: Honoraria, Research Funding; uniQure: Research Funding; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer (DMC): Honoraria; F. Hoffmann-La Roche Ltd/Genentech, Inc.: Honoraria; Sanofi: Honoraria; Spark (DMC): Honoraria; ICER: Membership on an entity's Board of Directors or advisory committees; WFH USA: Membership on an entity's Board of Directors or advisory committees; BCBS MAP: Membership on an entity's Board of Directors or advisory committees. Sanabria: F. Hoffmann-La Roche Ltd: Current Employment, Current holder of individual stocks in a privately-held company. Shapiro: Pfizer: Research Funding; OPKO: Research Funding; Octapharma: Research Funding; Novo Nordisk: Other: Advisory board fees, Research Funding, Speakers Bureau; Novartis: Research Funding; Kedrion Biopharma: Research Funding; Glover Blood Therapeutics: Research Funding; Genentech: Other: Advisory board fees, Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Bioverativ (a Sanofi company): Other: Advisory board fees, Research Funding; BioMarin: Research Funding; Takeda: Research Funding; Sangamo: Other: Advisory board fees, Research Funding; Sigilon Therapeutics: Other: Advisory board fees, Research Funding; Prometric BioTherapeutics: Research Funding; Agios: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal